TFF Pharmaceuticals Awarded Contract under DARPA’s Next-Generation Personalized Protective Biosystems Program for U.S. Warfighters
Company’s Thin Film Freezing platform to be developed for use in rapidly neutralizing chemical and biological threats at vulnerable tissue barriers to increase soldier protection and decrease the operational burden
TFF Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company focused on developing and commercializing innovative drug products based on its patented Thin Film Freezing (TFF) technology platform, today announced that Leidos, a leading Fortune 500 information technology, engineering, and science solutions and services leader, has awarded the Company a subcontract to participate in the Personalized Protective Biosystems (PPB) Program to develop next-generation chemical and biological protection for U.S warfighters and stability operators.
The PPB research program, overseen by the Defense Advanced Research Projects Agency (DARPA), will develop an integrated system that simultaneously reduces protective equipment needs while increasing protection for the individual against existing and future chemical and biological (CB) threats. This will be achieved through lightweight materials that protect the warfighter or stability operator from exposure to CB threats, while simultaneously providing a second layer of protection, at the tissue barrier, with bio-molecular, commensal organisms, or other technologies that protect the skin, eyes, and airway from CB threats. Successful PPB technologies could change how the military and public health communities perform in unpredictable threat environments.
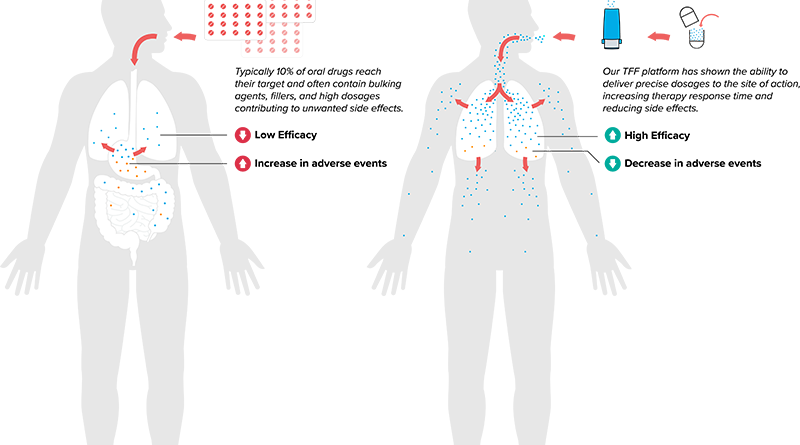
Under the 60-month, three-phase subcontract with Leidos, TFF Pharmaceuticals will utilize its Thin Film Freezing platform to formulate a series of countermeasures designed to neutralize chemical and biological agents at the site of vulnerable tissue barriers, including the skin, the eyes, and the respiratory system. Phase I of the program will include the development and validation of methods to quantify countermeasures and the formulation of countermeasures for delivery to the various tissue. Phase II will include the scale-up manufacturing of countermeasure formulations for preclinical studies, demonstration of the deliverability of countermeasure products, and meeting with the FDA to determine a path to GMP production of the countermeasure formulations, nonclinical safety testing, and a pathway to human clinical testing. Phase III will include plans for scale-up manufacturing for human safety trials.
“We are very pleased that Leidos selected our Thin Film Freezing platform to help develop the next generation of chemical and biological protective technologies for our frontline warfighters and stability operators,” said Glenn Mattes, President, and CEO of TFF Pharmaceuticals. “The Personalized Protective Biosystems program will develop groundbreaking technology and we are proud to be able to play a role in this program that will have a strategic impact on this country for years to come.”